|
 LACTOGG®’s probiotic strain has extensive research data documenting its specific and direct effect in acute gastroenteritis, especially that caused by rotavirus. Many clinical studies have demonstrated that taking GG probiotic at the first sign of diarrhoea, together with rehydration salt solutions, reduces the severity of the condition. In one clinical trial, the duration of diarrhoea in patients who took GG probiotic was found to be shortened by 50%. 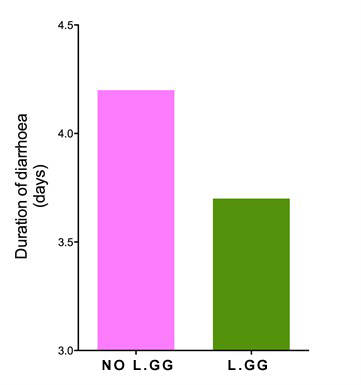 Lactobacillus GG shortens acute diarrhoea. [Guarino et al.1997] 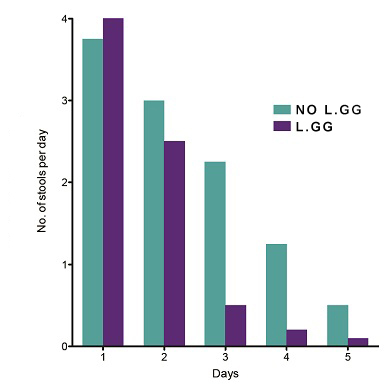 Average number of watery stools. [Guandalini et al. 2000] 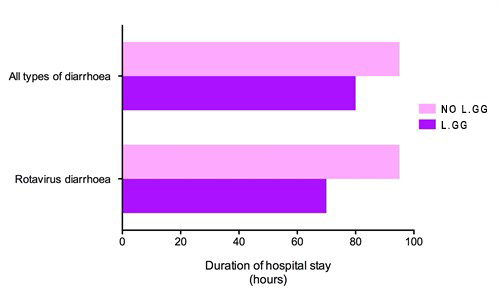 Duration of hospital stay in rotavirus-positive diarrhoea patients. [Guandalini et al. 2000] A medical team in Italy compared the effectiveness of 5 different brands of probiotics. Their findings, published in the British Medical Journal, were startling. The team found that most of the probiotic brands claiming to be effective in diarrhoea were actually ineffective. LACTOGG®’s probiotic strain however was effective and shortened diarrhoea by more than 1½ days. The doctors’ conclusion was: “Not all commercially available probiotic preparations are effective in children with acute diarrhoea.” Their advice was that we “should choose bacterial preparations based on effectiveness data”  The beneficial effects of GG probiotic in diarrhoea are so well established that major medical bodies have highlighted the use of GG probiotic in their guidelines on the treatment of childhood diarrhoea. The medical bodies include the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), European Society for Paediatric Infectious Diseases (ESPID), the Iberic-Latin American Guidelines Committee and the Academy of Medicine of Malaysia, College of Paediatrics.
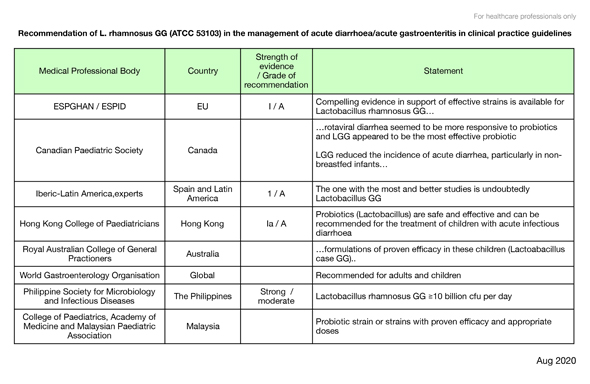
The following table lists a selection of professional bodies and medical societies which recommend the use of the GG probiotic in their guidelines.
Some hospitals, like the Cincinnati Children’s Hospital Medical Center in Ohio, USA, have fully adopted the use of GG probiotic as standard therapy for all children and teenagers admitted for acute diarrhoea. After a bout of gastroenteritis, GG probiotic’s ability to rapidly re-establish the damaged intestinal ecosystem and re-grow the intestinal surface ensures that nutrition is not interrupted. How should LACTOGG® be taken? Babies, children & adults: At least 1 capsule or 1 sachet a day, at the first sign of diarrhoea. If there is significant vomiting, you may prefer to dissolve the sachet powder into some rehydration solution or to take the capsule apart and just take the powdered contents with some rehydration solution. When the frequency of diarrhoea is high, repeated and increased doses of LACTOGG® may be taken, to promote colonisation. Continue LACTOGG® until diarrhoea has settled, and then for at least another one week to rebuild the intestinal surface and re-establish the disturbed intestinal bacteria. As long as there is diarrhoea, remember to continue taking oral rehydration salt solutions, to prevent dehydration. References: Canani RB et al.Effect of oral administration of Lactobacillus GG on the duration of diarrhea and on rotavirus excretion in ambulatory children.J Pediatr Gastroenterol Nutr 1997;24:469 Canani RB et al. Probiotics for treatment of acute diarrhoea in children - randomised clinical trial of five different preparations. BMJ 2007;335:340 Guandalini S et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhoea: a Multicenter European Trial. J Pediatr Gastroenterol Nutr 2000;30:54-60 Guarino A et al. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr 1997;25:516-519 Guarino A et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Ped Gastroenterol Nutr 2008;46:S81-S184 Guarino A et al. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases Evidence-based Guidelines for the Management of Acute Gastroenteritis in Children in Europe- Update 2014. J Pediatr Gastroenterol Nutr 2014;59(1):132-52 Gutierrez Castrellon P et al. An evidence based Iberic-Latin American guideline for acute gastroenteritis management in infants and prescholars. Anales de Pediatria 2010;72(3):220.e1-220.e20 Huang JS et al.Efficacy of probiotic use in acute diarrhea in children- a meta-analysis. Dig Dis Sci 2002;47(11):2625-2634 Isolauri E et al. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991;88(1):91-97 Isolauri E et al. Oral bacteriotherapy for viral gastroenteritis. Dig Dis Sci 1994;39(12):2595-2600 Majamaa H et al.Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis.J Pediatr Gastroenterol Nutr 1995;20:333-8. Parker MW et al.Rapid adoption of Lactobacillus rhamnosus GG for acute gastroenteritis. Pediatrics 2013;131:S96-S102 Pant AR et al.Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr 1996;42:165 Piescik-Lech M et al. Lactobacillus GG (LGG) and smectite versus LGG alone for acute gastroenteritis: a double-blind, randomized controlled trial. Eur J Pediatr 2013;172:247-253 Rautanen T et al.Management of acute diarrhoea with low osmolarity oral rehydration solutions and Lactobacillus strain GG. Arch Dis Child 1998;79:157-160 Raza S et al. Lactobacillus GG promotes recovery from acute non-bloody diarrhoea in Pakistan. Pediatr Inf Dis J 1995;14:107-111 Szajewska H et al. Meta-analysis:Lactobacillus GG for treating acute gastroenteritis in children- updated analysis of randomised controlled trials. Aliment Pharmacol Ther 2013:38:467-476 Szajewska H et. al. Use of Probiotics for Management of Acute Gastroenteritis- A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2014;58(4):531-539 |